I Preparation of Equipment and Reagents
1. Instrument (tumor tissue)
ultra-clean bench or biosafety cabinet, water bath, one disposable 100 mm Petri dish, several sterilized scissors and forceps, sterilized 100mL shaking flask, electronic pipette, disposable centrifuge tubes of 15/50mL, some disposable 50mL syringes and 0.22μm filters, sterilized 100ml reagent bottle, centrifuge, 5ml disposable pasteur pipet, 75% alcohol and watering can,0.4% trypan blue and blood counting chamber, crushed ice, foam box
2. Instrument(the digestion of solid-organ)
ultra-clean bench or biosafety cabinet, water bath, peristaltic pump(LongerPump,YZ1515X), push-type electric waste liquid cylinder, one disposable 100mm Petri dish, latex tube (sterilized, length 168cm, specification filled with 14ml liquid), sterilized scissors and forceps, sterilized arterial clip, sterilized hemostatic forceps, disposable infusion needle (black 0.7*25), electronic pipette, disposable 15/50mL centrifuge tubes, disposable 50mL syringes and 0.22μm filters, 100mL sterilized reagent bottle, centrifuge, foam plate, waste liquid tray, fixed needle, disposable 5 ml pasteur pipet, 75% alcohol and watering can, 0.4% trypan blue and blood counting chamber, crushed ice, foam box, freshly prepared 6% sodium pentobarbital sodium.
3. Kits, Reagents and Consumables
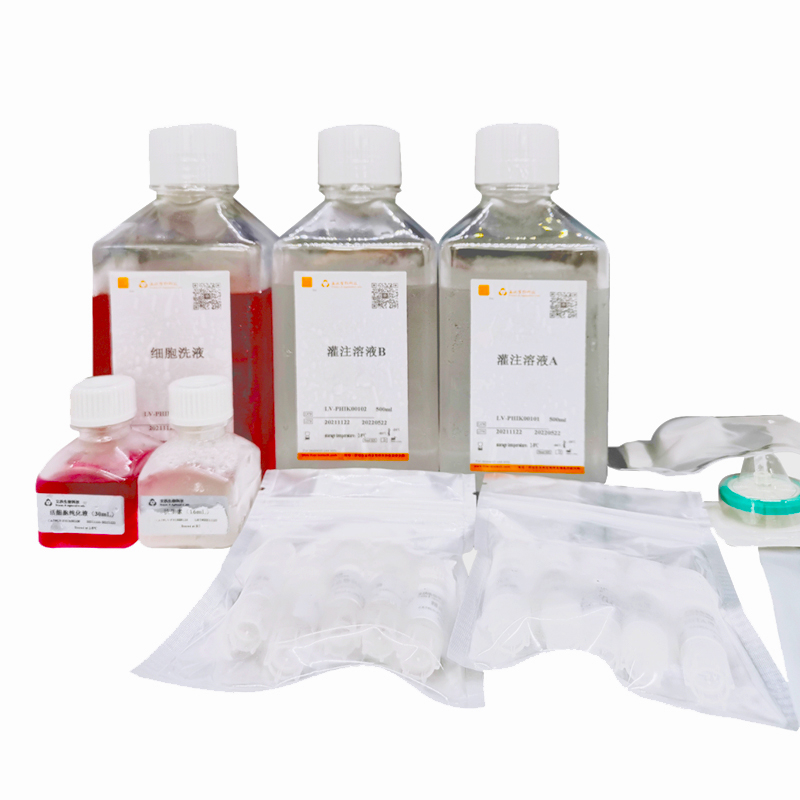
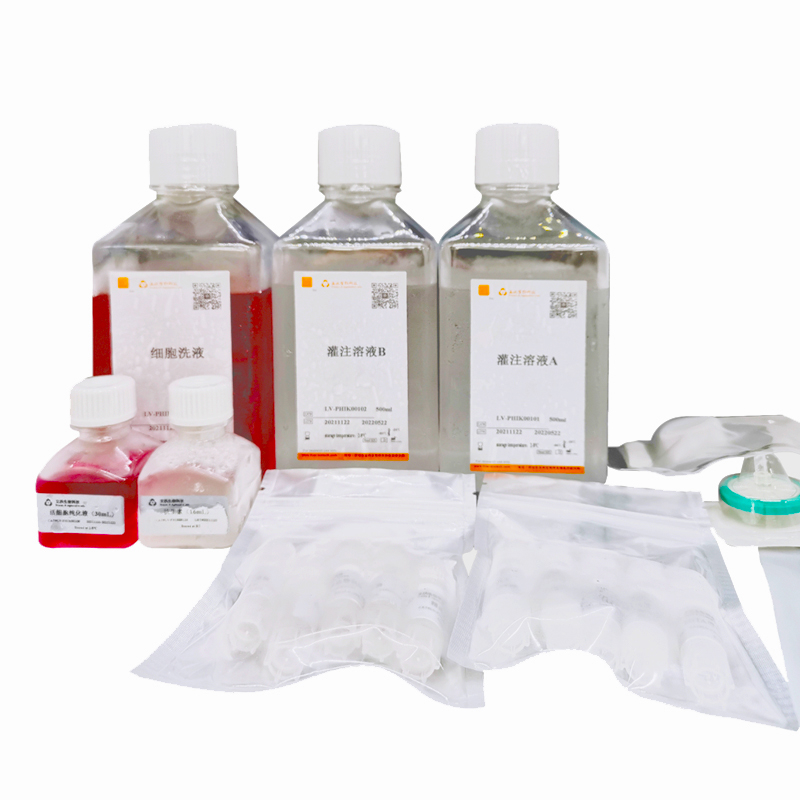
TABLE 1 Kit List
Product | Type | Spec. | Number |
Perfusion solution A | LV-PHIK00201 | 500mL/bottle | 1 bottle |
Perfusion solution B | LV-PHIK00202 | 500mL/bottle | 1 bottle |
Washing buffer | LV-PHIK00203 | 500mL/bottle | 1 bottle |
Purification buffer for viable cells | LV-PHIK00104 | 30mL/bottle | 1 bottle |
0.22µm filter | LV-PHIK00105 | 5/pack | 1 pack |
20mLsyringe | LV-PHIK00106 | 5/pack | 1 pack |
70µm cell strainer | LV-PHIK00107 | 5/pack | 1 pack |
Selected Collagenase | LV-PHIK00108 | 5vial/case | 1case |
EGTA Stock (100x) | LV-PHIK00109 | 1.1mL/vial | 5vials |
Antibiotic | LV-PHIK00110 | 16mL/ bottle | 1 bottle |
II Reagent Preparation (ready-to-use)
1. Working solution of perfusion solution A(LV-PHIK00201):Under sterile conditions, take 100mL of perfusion solution A to 100mL of sterilized reagent bottles, add 1mL of EGTA mother liquor (LV-PHIK00109) and 1mL of antibiotics (LV-PHIK00110), and put them at 4 °C for backup. Note: Theoretically, 100 mL of working solution of perfusion solution A is sufficient for pretreatment of 1-3 g of tissue or organ, which can be further optimized in skilled cases.
2. Working solution of perfusion solution B(LV-PHIK00202):Under sterile conditions, take 100 mL of perfusion solution B and take a selected collagenase (LV-PHIK00108). Fully dissolve selected collagenase through 20 mL of perfusion solution B and filter with a 0.22 μm filter (LV-PHIK00105). Finally, add 1 mL of antibiotic (LV-PHIK00110) and put it at 37 °C for backup. Note: Theoretically, 100 mL of working solution of perfusion solution B is sufficient to digest 1-3 g of tissue or organ, which can be f of Tumor Tissue further optimized in the case of proficiency.
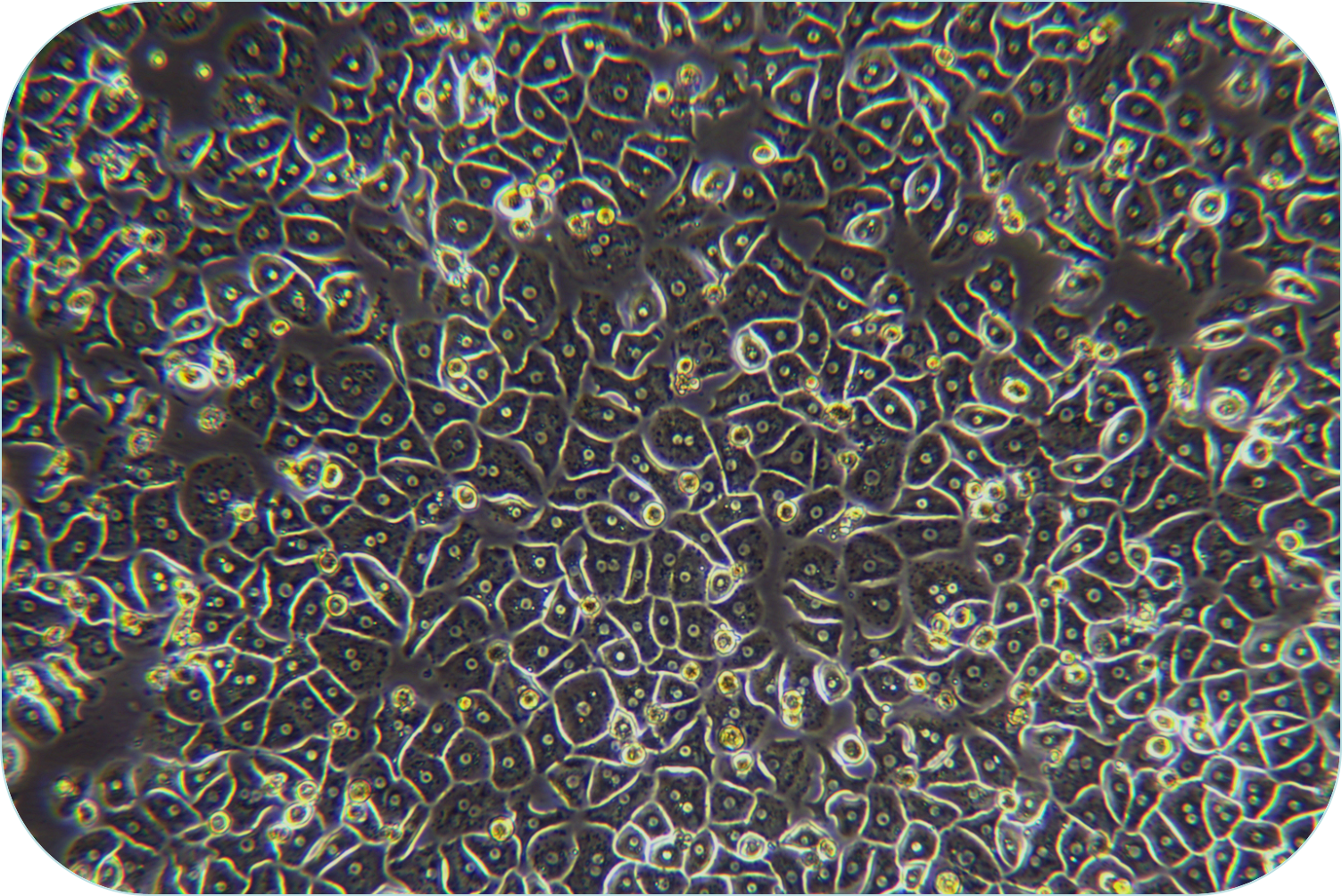
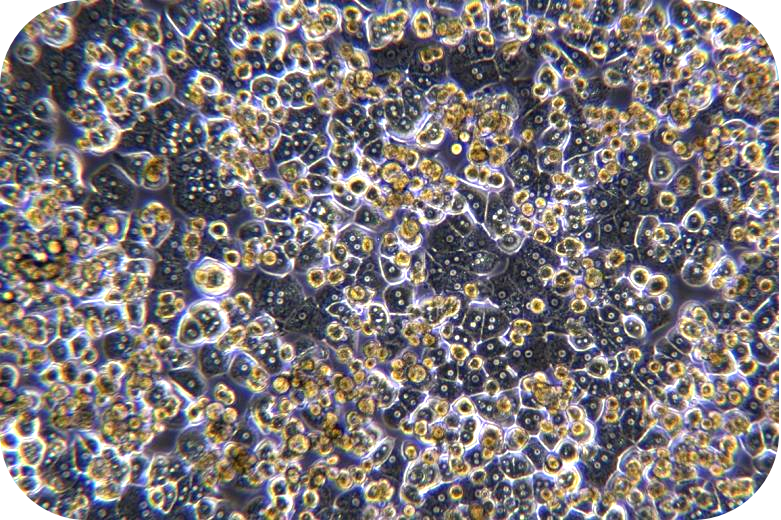
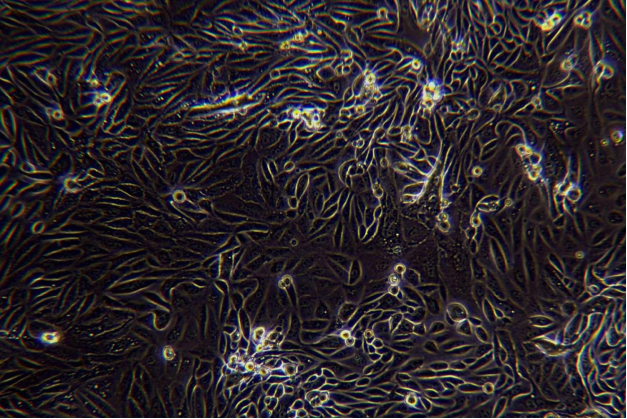
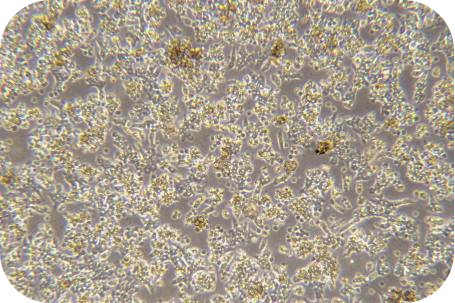
III Digestion (take liver cancer tissue as an example)
1. The sample should be placed in a 100 mm Petri dish and be added to working solution of 10 mL of ice-cold perfusion solution A. The envelope, fat, blood, etc. can be removed with sterile scissors and forceps, and wash 3 times.
2. Metastasize the tumor tissue to a new 50 mL centrifuge tube and add 10 ml of ice-cold working solution of perfusion solution A. Then, cut the tissue block into 1mm size of tissue blocks. Add ice-cold working solution of perfusion solution A to the volume of 20 mL. After that,centrifuge at 100 x g for 5 min, go supernatant and wash 3 times.
3. Add 20 mL of working solution of perfusion solution B (perfusion solution B (LV-PHIK00202) is also available). Mix well upside down, centrifuge at 100 x g for 5 min, and remove the supernatant (remove EGTA of working solution of perfusion solution A).
4. Add 20mL of working solution of perfusion solution B. Invert and mix well, transfer to 100mL shaking flask. Add the remaining working solution of perfusion solution B. Place it on a shaking flask at 37 °C after sealing. Then, speed 120rpm and digest 0.5-2hours.To avoid low cell activity due to over-digestion, it is recommended to observe the shaking flask every 15 min until 80% of the tissue blocks are gone.
5. Remove the shaking flask and add EDTA mother liquor (LV-PHIK00109) to the amount of 1 mL of working solution of perfusion solution B /10 μL of EDTA. Then, stop digestion for five min.
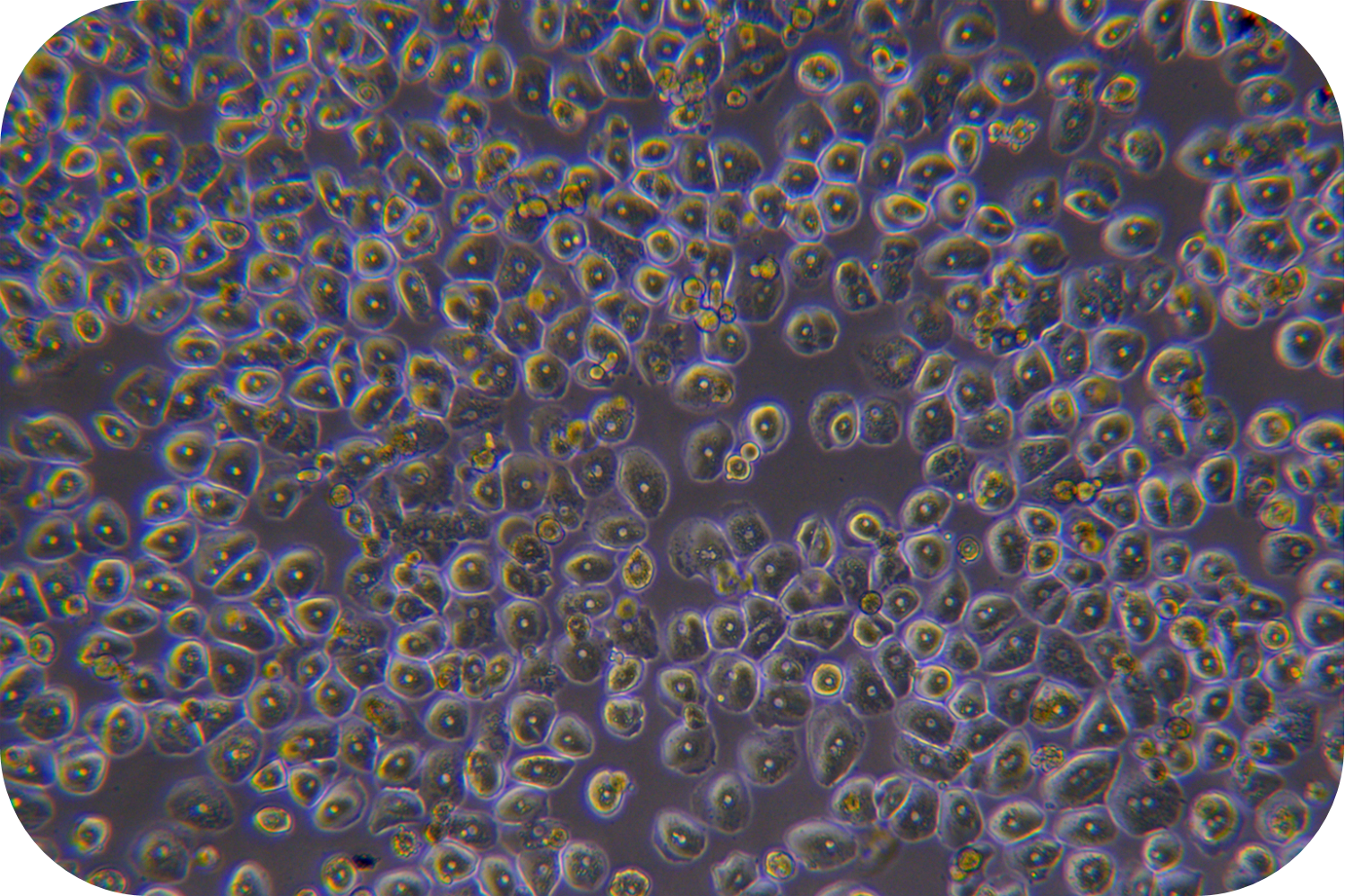
6. Trypan blue detects cell viability for further cell sorting and purification. If the cell activity rate is less than 60%, the live cell purified solution (LV-PHIK00104) can be used. The recommended steps are as follows:
(1) Centrifuge of 50 g at 4 °C for 5 min and go supernatant. If 1-3 g of tumor tissue cells are purified, resuspend the cells with 10 mL of purified solution of live cell (LV-PHIK00104) in a 15 mL centrifuge tube. Then carefully add 5 ml of washing buffer (LV-PHIK00203) along the wall to the centrifuge tube (Do not disturb the lower layer, significant layering can be seen after addition).
(2) Centrifuge of 800 × g at 4 °C for 20 min and reduce acceleration to 1. After centrifugation, aspirate the turbid band (living cell layer) between purified solution of live cell (LV-PHIK00104) and washing buffer (LV-PHIK00203). Then, transfer it to a new centrifuge tube of 50mL. Then, add 2 volumes of washing buffer (LV-PHIK00203) and mix gently up and down. Centrifuge at 100 × g of 4℃ for 5 min (adjustable according to cell type).
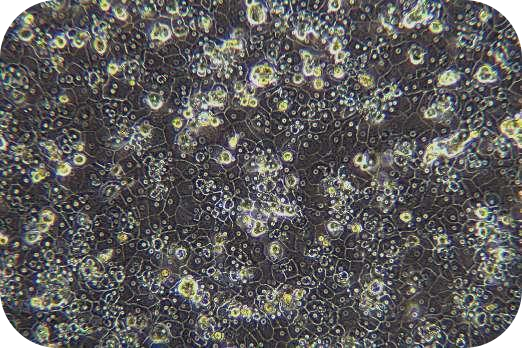
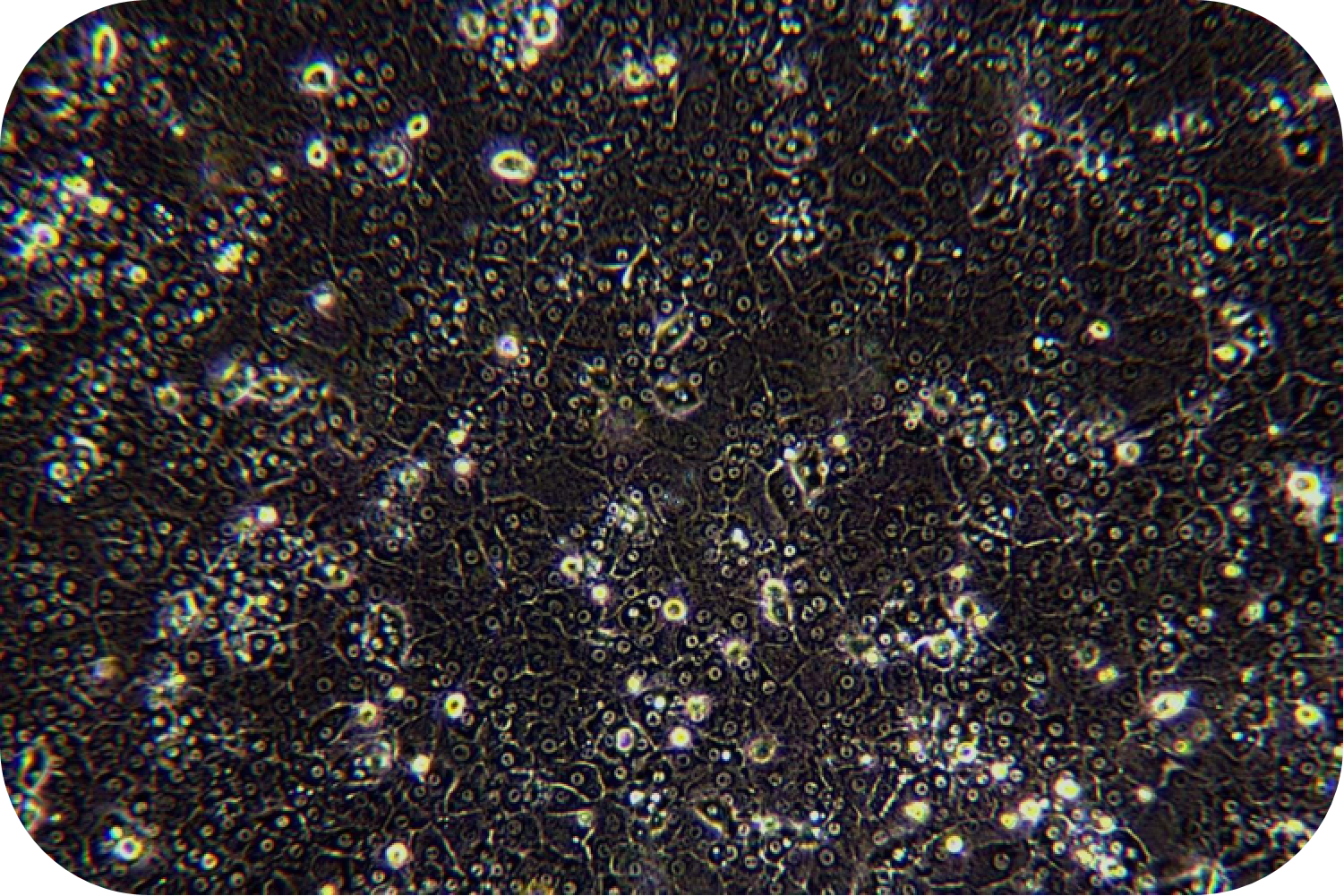
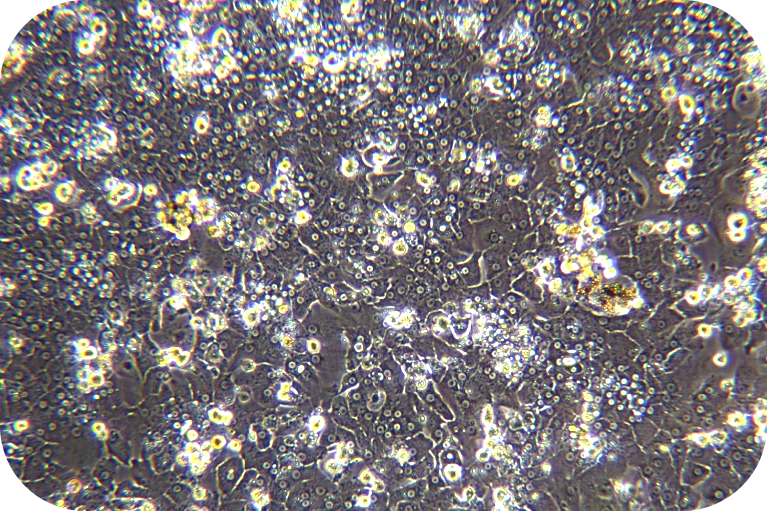
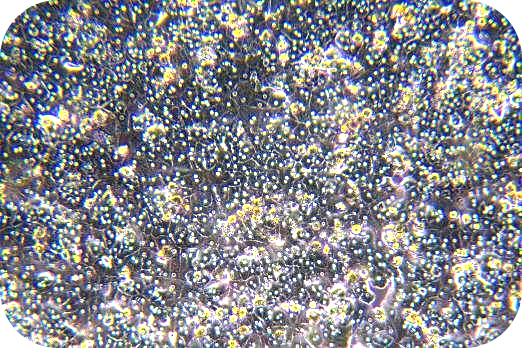
(3) Cell viability can be measured by Trypan blue and proceeds the further downstream.
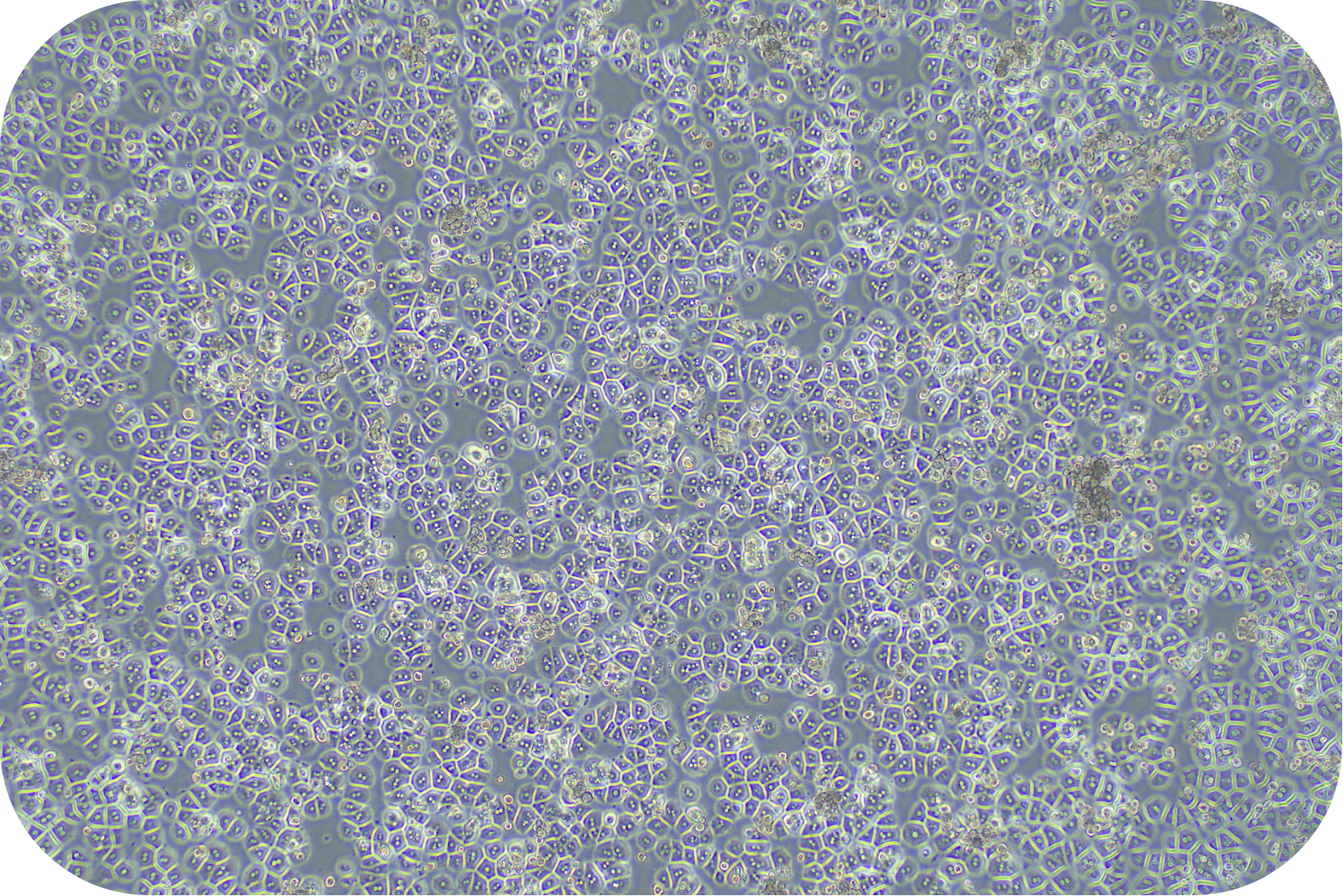
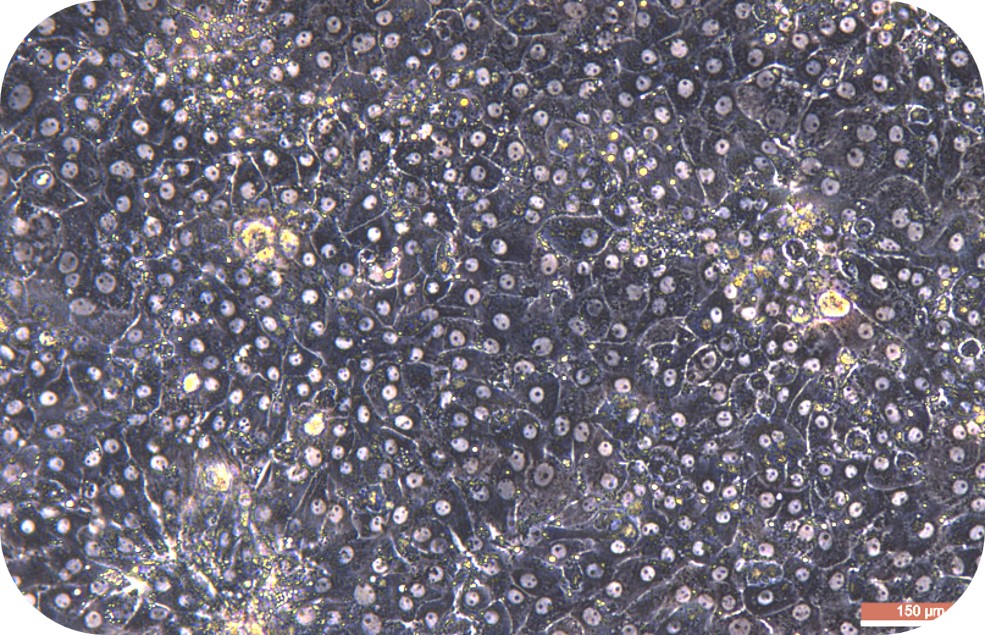
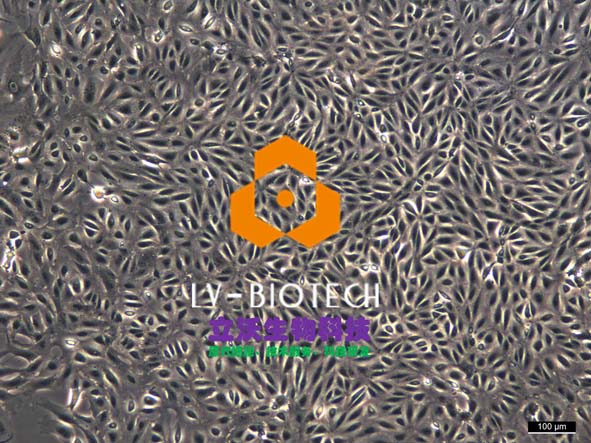
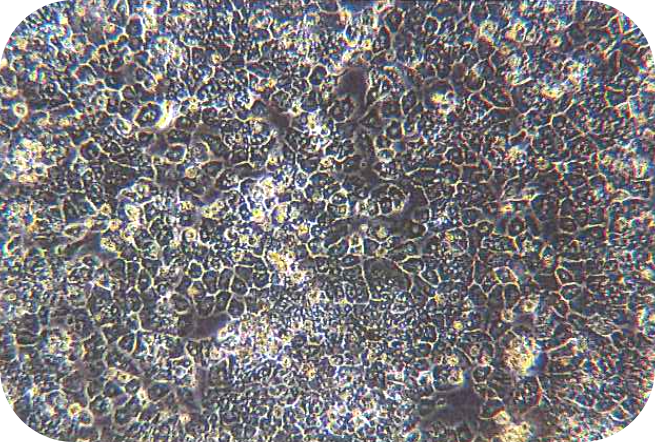
IV Digestion of Solid Organ (take mouse liver as an example)
1. Pick up the surface of the mouse's abdomen with forceps. Cut a small piece from the middle, then cut from the sides until the entire inner layer of skin is exposed. Then, skin is cut off;
2. Replace the sterile scissors and forceps with the inner layer of the abdomen and cut a piece from the middle to the sides until the entire abdominal cavity is exposed. Fix the skin on both sides as well as the inner layer of the skin with a fixing needle to prevent the fur from contaminating the liver cells;
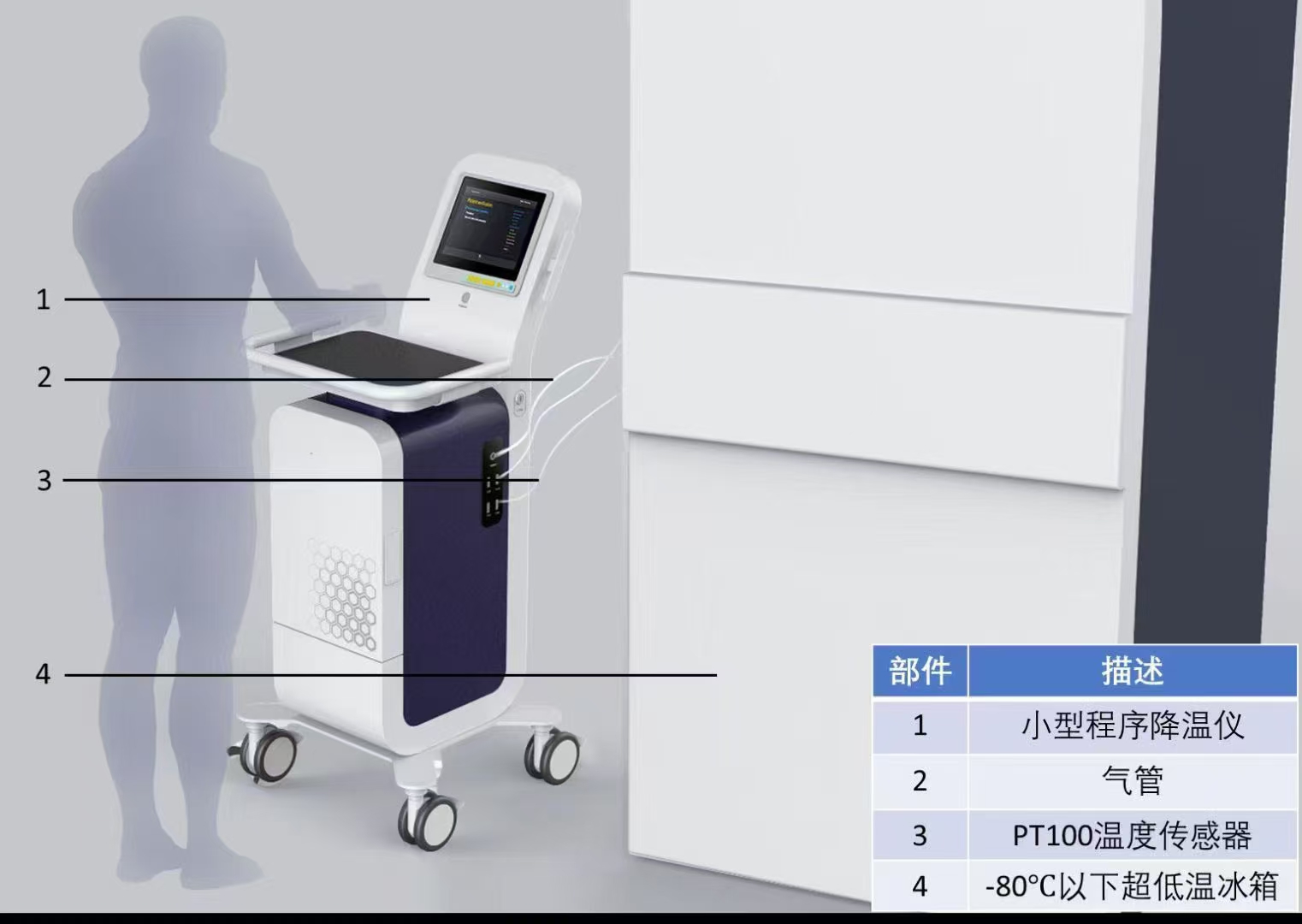
3. Gently turn the stomach and intestines to the right with forceps. Expose the inferior vena cava and turn on the peristaltic pump (at this time the flow rate is 1r/min). Then, the injection needle (linking the perfusion solution, the entire perfusion device has been drained of air) is inserted into the lower vena cava to begin perfusion. If the liver is whitened, the hepatic portal vein should be immediately cut. Slowly increase the perfusion speed to 7-9 mL/min, the flow rate can be increased by 1-2 ml/s (it is recommended to let the assistant adjust the flow rate). And cut the heart, with hemostatic forceps or arterial clamp to clamp the superior vena cava. At this time, the hepatic portal vein is clamped to show that the liver is more likely to expand and stand up, indicating that the insertion position is correct and the perfusion is successful.
4. Perfuse mice with 70 ml of working solution of solution A for about 6-8 min and perfuse 70 ml of working solution of solution B for about 6-8 min. During the perfusion of solution B, the hepatic portal vein needs to be clamped 5 times for about 2-5 seconds each time, so that the liver is expanded. The increased pressure in the liver can help the digestion of the liver for improving the yield.
5. Perfuse the digestive solution until the liver loses its elasticity, and press the liver with forceps. Then, the liver will form a noticeable imprint indicating that the digestion is complete.
6. Dissociate the liver rapidly (within 1-2 min) from the animal and transfer to a culture dish containing 10 mL of working solution of perfusion solution B. Then, tear the tissue apart, and carefully shake the cells off. If digested adequately, a sufficient amount of washing buffer can be added in time. (At this point, all steps involving hepatocyte processing must be sterile and handled gentle, at which point the hepatocytes are very fragile and susceptible to shear damage.) Add a sufficient amount of pre-chilled washing buffer (total volume is 30 ml). And, the cell suspension should be over a 70 μm of cell strainer (bottom should be prewetting).
7. Centrifuge at 50 g of 4 °C for 5 min. Pre-chill the washing buffer (30 ml/1-2 mouse hepatocytes) and resuspend cells. Then, gently blow out the cells, and have a repeated centrifuge for 2 times.
8. Pre-chill washing buffer and resuspend cells (about 30 ml/1-2 mouse hepatocytes). Count with trypan blue (trypan blue: cells = 1:9).
9. Cell purification: If the viability is too low (less than 80%), purification treatment should be required.
(1) Centrifuge at 50 g of 4 °C for 5 min and go supernatant. If purifying 1 mouse hepatocyte, resuspend the cells with 5 mL purification solution of live cell (LV-PHIK00104) in a 15 mL centrifuge tube. Then carefully add 5 ml of washing buffer (LV-PHIK00103) along the tube to the centrifuge tube (Do not disturb the lower layer, significant layering can be seen after addition).
(2) Centrifuge at 4 °C of 800 × g for 20 min and reduce acceleration to 1. After centrifugation, aspirate the turbid band (living cell layer) between purified solution of live cell (LV-PHIK00104) and washing buffer (LV-PHIK00103). Then, transfer it to a new 50 mL centrifuge tube and add twice the volume of washing buffer (LV-PHIK00103), gently invert up and down to mix well. Centrifuge at 4 °C of 50 × g for 5 min.
(3) Remove the supernatant, resuspend the cells with plating medium of mouse (LV-WEP006 recommended) and cell count to determine the effect of purification. Theoretically, cell viability can reach more than 85%.