Transport Mode
Liquid Nitrogen Transportation
1、Adsorptive Liquid Nitrogen Transportation ,No free liquid nitrogen ,White vapor emission indicates normal operation.
2、Temperature monitoring devices should closely monitor the temperature inside the tank during transportation. If any abnormalities are detected, you can copy the PDF and Excel data from the temperature recorder. (One end of the temperature monitoring device can be unplugged to reveal a USB connector. Simply insert it into a computer to copy the data. If you encounter any difficulties, you can contact the sales staff and technical support for assistance.)
3、Alloy Combination Lock Designed to ensure no third-party access to the LN₂ tank or cell samples during transportation from dispatch to receipt, thereby safeguarding cell integrity.
4、GPS Tracker Enables real-time tracking of cell transportation routes to prevent loss.
5、Liquid Nitrogen Tank, temperature monitoring device, alloy combination lock, and GPS tracker are returnable components. Please store them properly and avoid damage; failure to comply will result in blacklisting.
Reference Citation
pass over.
Scope of Application
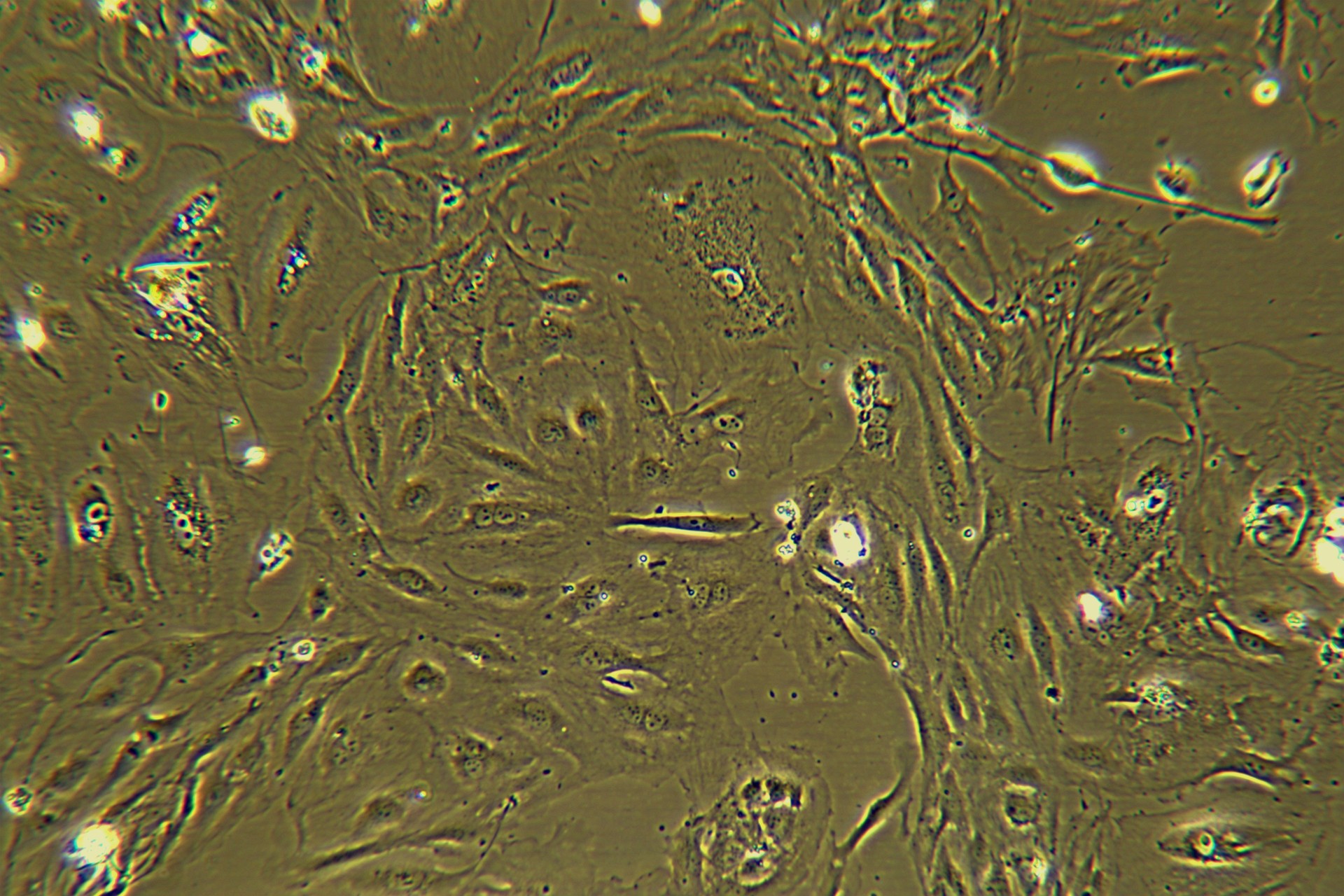
Hepatic stellate cells (HSCs) hold significant value in biomedical research, and their applications can be summarized into the following core directions:
1.Mechanisms of Liver Fibrosis and Cirrhosis
Hepatic stellate cells (HSCs) are the central effector cells in liver fibrosis. Under pathological conditions, HSCs are activated and transdifferentiate into myofibroblasts, secreting excessive extracellular matrix (ECM) components (e.g., collagen I/III) that drive fibrogenesis. Researchers investigate the molecular mechanisms of fibrosis by: In vitro models: Utilizing activated HSCs (e.g., the human LX-2 cell line) to analyze cytokine secretion (e.g., TGF-β, IL-6), ECM composition, and signaling pathways (e.g., AKT phosphorylation).
Genetic models: Employing gene-knockout animals (e.g., Plvap-null mice) to dissect HSC-specific molecular networks.
Functional studies: Characterizing HSC contractility to elucidate mechanisms of sinusoidal hypertension in cirrhosis.
2. Research on Metabolic Regulation and Energy Balance
Hepatic stellate cells (HSCs) regulate hepatic metabolic substrate preferences through specific proteins such as PLVAP (Plasmalemma Vesicle-Associated Protein). For instance, during fasting, PLVAP in HSCs modulates fatty acid esterification and oxidation, determining whether the liver prioritizes fatty acids or carbohydrates for energy production. PLVAP-knockout mice exhibit enhanced glucose metabolism capacity and activated insulin signaling pathways, offering novel insights into metabolic disorders such as diabetes and non-alcoholic fatty liver disease (NAFLD). Furthermore, the vitamin A storage capacity of HSCs has been leveraged to study lipid-soluble vitamin metabolism and its dysregulation in chronic liver diseases.
3. Models of Liver Regeneration and Cell-Cell Interactions
Hepatic stellate cells (HSCs) secrete growth factors (e.g., HGF and VEGF) to promote hepatocyte proliferation and angiogenesis, playing a dual role in liver injury repair. In research, co-culture systems (e.g., HSCs with hepatocytes or liver progenitor cells) are widely used to mimic the regenerative microenvironment. These models enable analysis of HSC-mediated effects on hepatocyte functions, such as albumin secretion and drug-metabolizing enzyme activity (e.g., CYP450 enzymes). Additionally, such systems help elucidate interaction mechanisms between HSCs and immune cells (e.g., macrophages), dissecting the balance between inflammation and regeneration during liver repair.
4. Drug Development and Anti-fibrotic Therapy Screening
Immortalized HSC cell lines (e.g., LX-2, HSC-Li) are indispensable tools for anti-fibrotic drug screening. Researchers evaluate candidate compounds through in vitro assays that measure their inhibitory effects on HSC proliferation, α-smooth muscle actin (α-SMA) expression, and collagen secretion, followed by in vivo validation in animal models (e.g., CCl₄-induced fibrosis). For instance, small-molecule inhibitors targeting HSC activation pathways (e.g., TGF-β/Smad signaling, PDGF receptor tyrosine kinase) and gene therapies (e.g., siRNA against Col1a1) have advanced to the preclinical research stage.
5. Research on Tumor Microenvironment and Cancer Progression
Activated hepatic stellate cells (HSCs) participate in the remodeling of the liver cancer microenvironment and the invasion/metastasis of tumor cells by secreting matrix metalloproteinases (MMPs) and inflammatory factors. In scientific research, co-culture models of HSCs and liver cancer cells are frequently utilized to investigate their regulatory roles in tumor growth, angiogenesis, and immune escape, thereby providing new strategies for liver cancer treatment.
Due to biannual updates of our product instruction manual, the following procedures are for reference only.
The most recent version included with the product shall prevail.
II Reagents and Materials
- Hepatic stellate cells(Cat# LV-HSC001)
- Recovery medium(Cat#LV-Rec001)
- Stellate cell culture medium (Cat# LV-HSCM001)
-Crushed ice and ice boxes
- Sterile centrifuge tube of 15 ml
- Disposable pipette
- Wide-mouth pipet tip (The tip of a normal one is cut off and sterilized)
- Pipette
-Thermostat water bath (Preheat at 38℃)
- Refrigerated horizontal centrifuge (With horizontal rotor,it is possible to centrifuge 15 ml of centrifuge tubes)
- Biosafety cabinet
- 37 °C/5% CO2 incubator
- 75% alcohol
III Resurgence and Plating of Cells
1. Insert the 15 ml centrifuge tube into an ice box containing enough crushed ice and sterilize it UV for 15 min. Precool centrifuge.
2. The recovery medium should be placed in crushed ice for sufficiently precooling, and the plating medium should be placed in a 38 °C thermostat water bath to be fully preheated.
3. Quickly transfer the frozen hepatic stellate cells from the refrigerated position to a thermostat water bath of 38℃. Then,immerse them in as much water as possible at 38°C and shake clockwise. Please make sure that the cap of the freezing tube is kept above the water.
4. Thaw the freezing tube for about 90-120s, until only a small amount of crushed ice floats in it.
5. Sterilize the freezing tube with 75% alcohol and transfer it to a biosafety cabinet.
6. Resuspend the cells with a wide-mouth pipet tip (gently blow 2 times) and transfer to a pre-cooled centrifuge tube of 15 ml.(Note: if there are more cells left on the freezing tube and the pipette tip, aspirate 1 ml of resuscitation medium to clean the them.
7. Add prechilled recovery medium to the cell suspension dropwise and add 10 ml of recovery medium to every 1 ml of cell suspension (Note: the first 3 ml should be added dropwise and shaken slightly, and the next 7 ml can be accelerated). Finally, slightly upside down 1 time to mix well.
8. Centrifuge of 600 × g at 4 °C for 5 min, remove the supernatant and resuspend with plating medium.
9. The pelleted cells should be resuspended with hepatic stellate cell culture medium and set the volume to 4 ml. The viability and total amount of hepatic stellate cell can be determined by trypan blue exclusion.
10. Cells should be seeded into a Petri dish at the density of 3×104cells/cm2, shaken well and cultured in a 37 °C/5% CO2 incubator. Change the solution after 24h.
IV Culture and Passage of Cell
1. The cells can be passaged when the degree of fusion reaches 80%.
2. Put the medium, PBS and pancreatin into thermostat water bath of 37℃ for preheating. And wipe it with 75% alcohol before placing in the ultra-clean table.
3. Aspirate the old culture solution and add a small amount of PBS to wash the cells. In addition, add an appropriate amount of pancreatic enzyme so that the amount of pancreatic enzyme can cover the cells. Incubate the cells at 37°C for 2-3 min and observe them under the microscope.
4. Discard the pancreatic enzyme when the intercellular space of the adherent cell becomes larger and the cells tend to be rounded but have not yet floated. After that, fresh culture medium should be added and the cell flask should be shaken to terminate the action of pancreatin. Then, carefully blow the adherent cells with a pipette to make a cell suspension (Note:Pay attention to the force of the blowing to avoid generating a large number of bubbles).
5. Centrifuge of 600 × g at 4 °C for 5 min, remove the supernatant and resuspend with the culture medium.
6. Inoculate the cell suspension into a new flask/plate at the density of 3×104cells/cm2 and place it in a 37 °C/5% CO2 incubator to observe the adherent growth every other day.
Post-Thaw Cells Must Not Undergo Refreezing.
V Customer Service
If you find any quality problems with the product, please collect the original data and contact the company's salesmen or technical support at the first time. The company ensure after-sales service. Every laboratory has different conditions, different operating habits, different proficiency, and objective factors in experimental failure. If the operation is not strictly in accordance with the instructions or exceeds the time limit of after-sales, the company does not do after-sales. Please understand and support us.
Validity period and raw data provided:
Resuscitation problems: Within 24 hours of resuscitation, trypan blue staining or PI staining should be provided.
Pollution problems: Within 96 hours of resuscitation, microscope photographs of differences should be provided.
Purity issues: Within one month, immunofluorescence or flow cytometry results should be provided
VI Contact Number
Tel:0755-28284050
Technical Support: 19902901483 (Dr. Zhou)