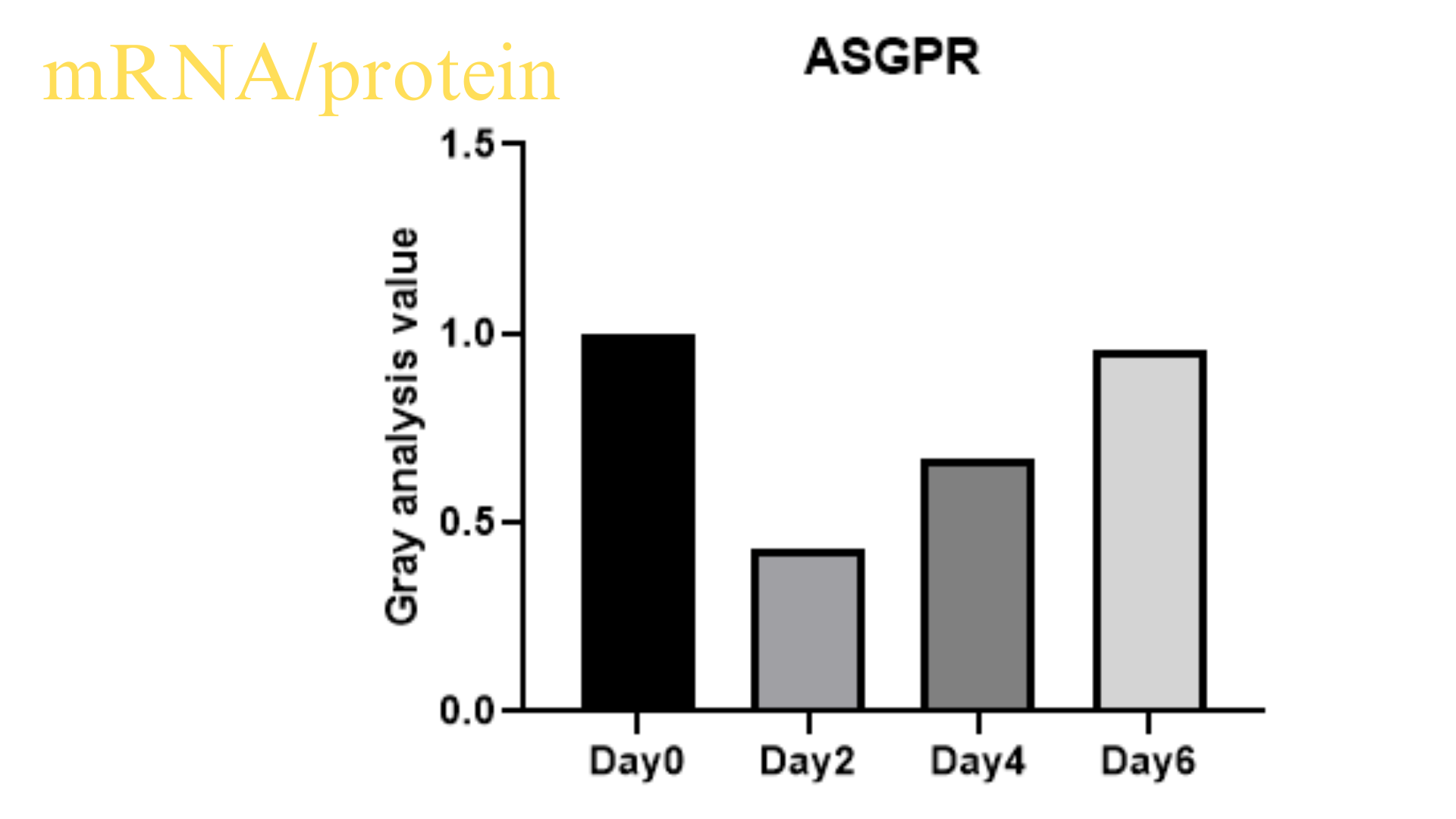
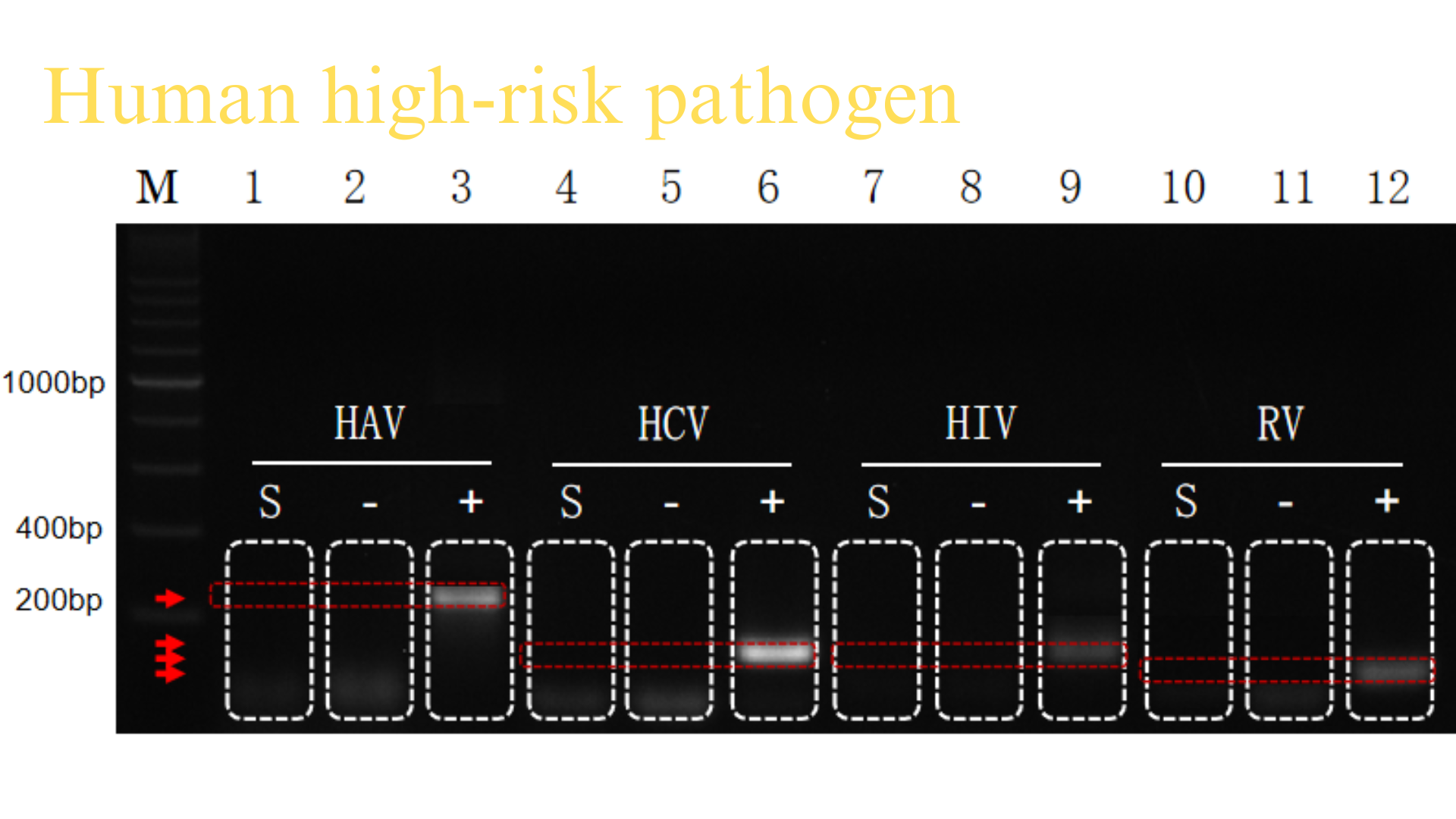
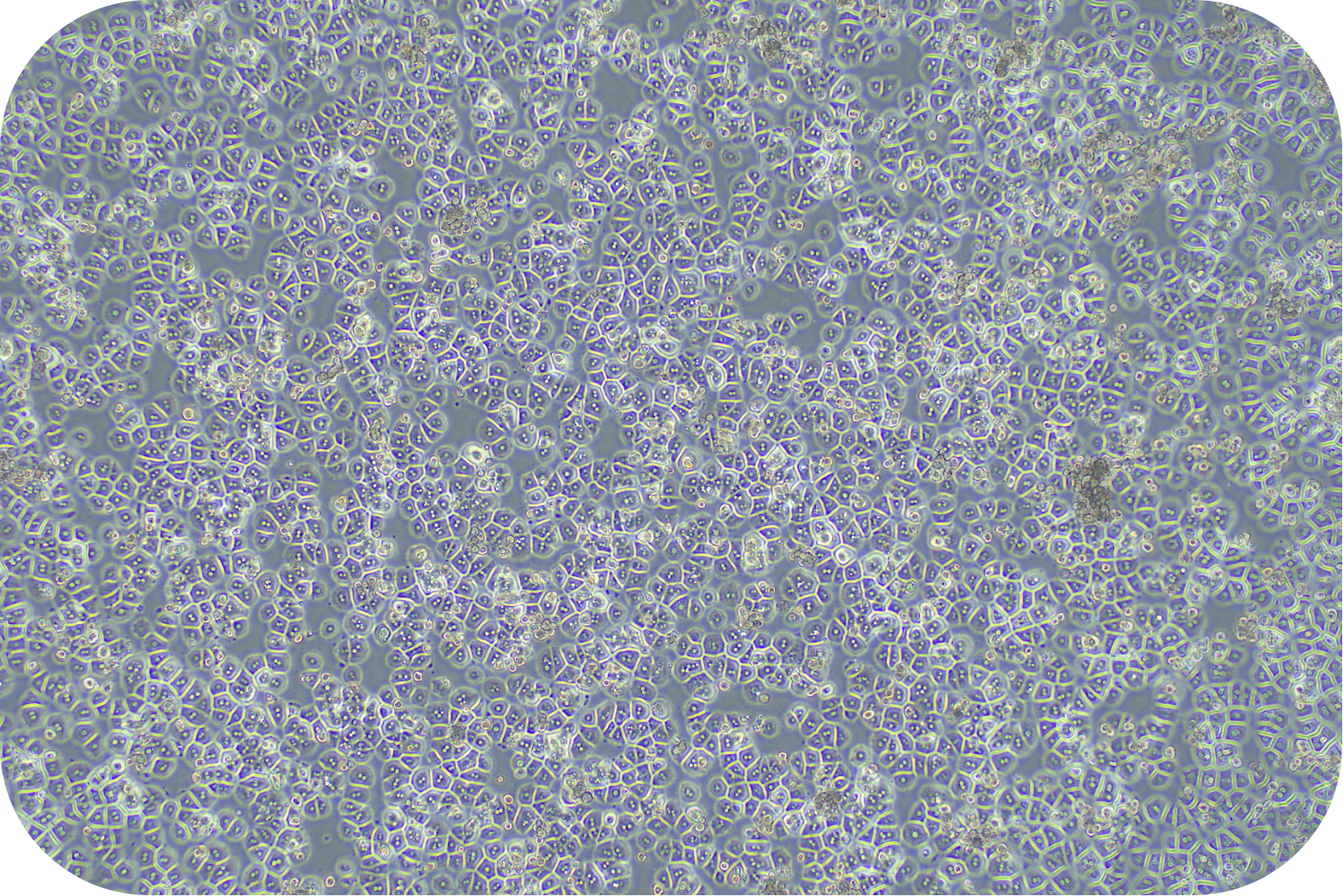
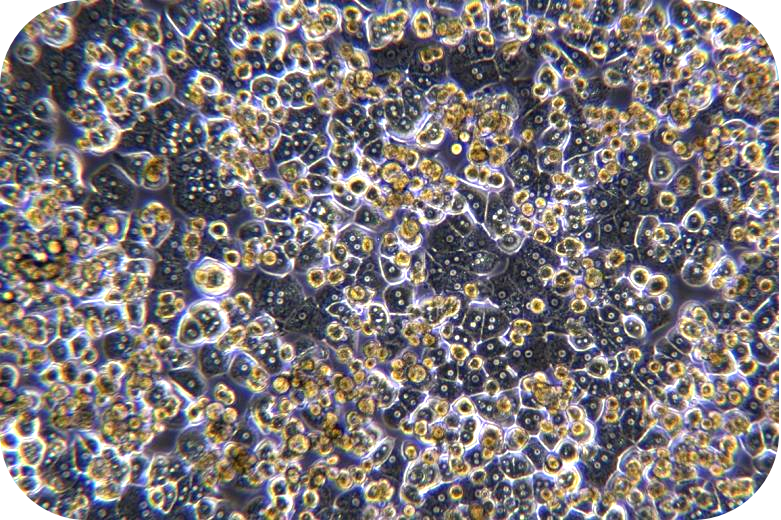
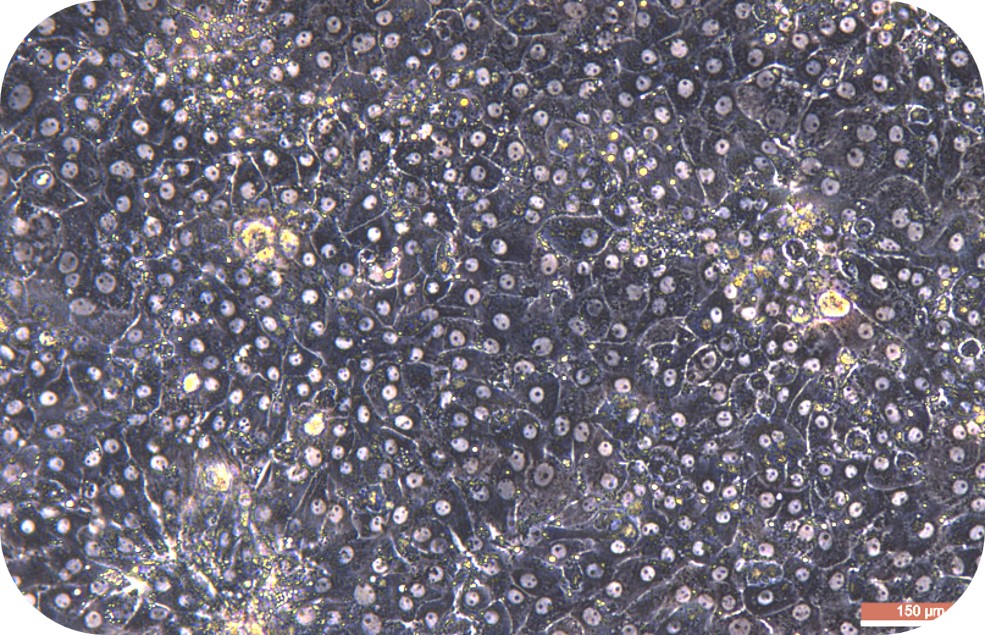
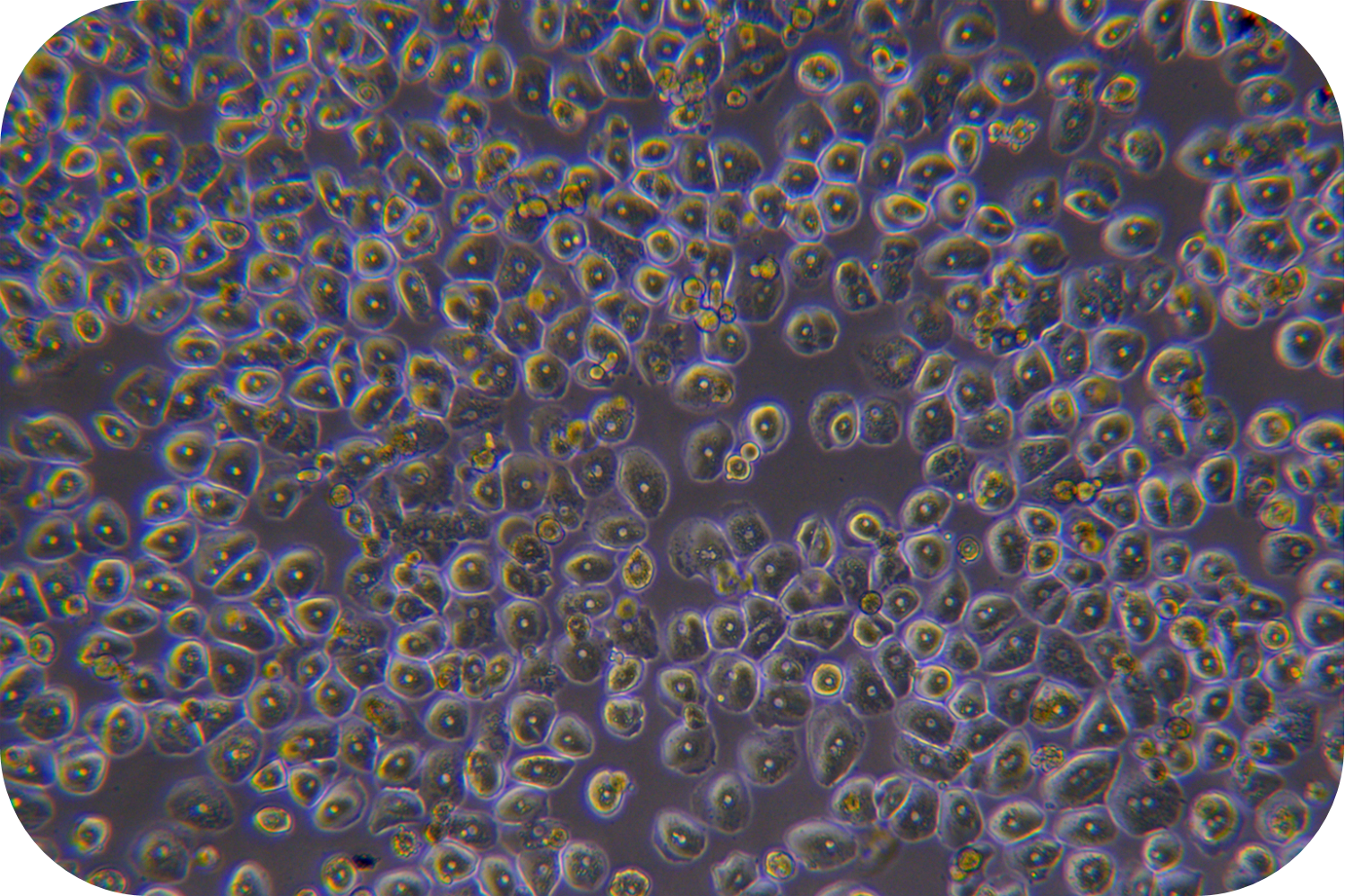
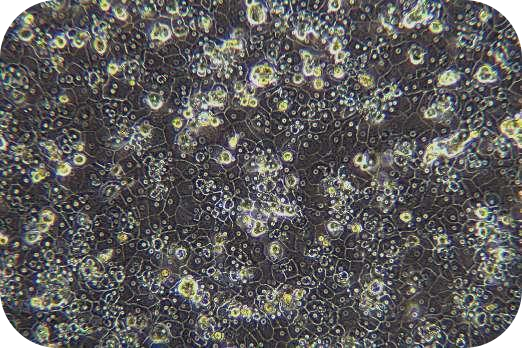
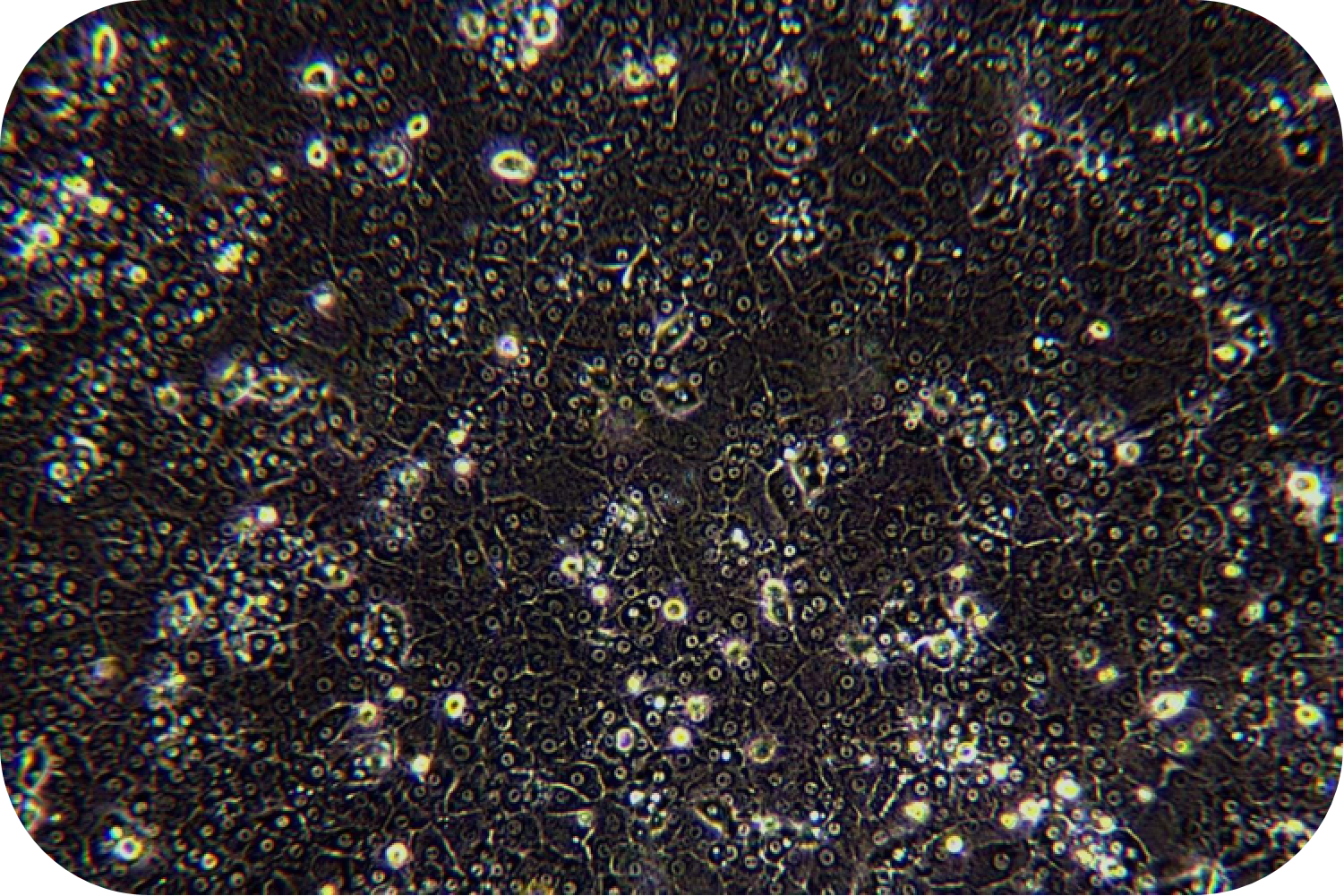
Transport Mode
Liquid Nitrogen Transportation
1、Adsorptive Liquid Nitrogen Transportation ,No free liquid nitrogen ,White vapor emission indicates normal operation.
2、Temperature monitoring devices should closely monitor the temperature inside the tank during transportation. If any abnormalities are detected, you can copy the PDF and Excel data from the temperature recorder. (One end of the temperature monitoring device can be unplugged to reveal a USB connector. Simply insert it into a computer to copy the data. If you encounter any difficulties, you can contact the sales staff and technical support for assistance.)
3、Alloy Combination Lock Designed to ensure no third-party access to the LN₂ tank or cell samples during transportation from dispatch to receipt, thereby safeguarding cell integrity.
4、GPS Tracker Enables real-time tracking of cell transportation routes to prevent loss.
5、Liquid Nitrogen Tank, temperature monitoring device, alloy combination lock, and GPS tracker are returnable components. Please store them properly and avoid damage; failure to comply will result in blacklisting.
Reference Citation
1、CHEN Yi, TANG Dan, WU Hongping, et al. Assessment of long-term functional maintenance of primary human hepatocytes to predict drug-induced hepatoxicity in vitro[J]. Archives of Toxicology, 2021, 95(6): 1-26. DOI: 10.1007/s00204-021-03050-y.
2、Youquan Zhong # 1 2, Chuanjian Wu # 1, Zaichao Xu 1, Yan Teng 1, Li Zhao 1, Kaitao Zhao 1, Jingjing Wang 1, Wen Wang 3, Qiong Zhan 1, Chengliang Zhu 4, Xinwen Chen 5, Kaiwei Liang 3, Xiaoming Cheng 1 6 7, Yuchen Xia 1Hepatitis B Virus Core Protein Is Not Required for Covalently Closed Circular DNA Transcriptional Regulation. J Virol. 2022 Nov 9;96(21):e0136222. doi: 10.1128/jvi.01362-22. Epub 2022 Oct 13.
3、Lin Xu 1, Qianwen Zhao 1, Jiao Luo 1, Wanli Ma 1, Yuan Jin 1, Chuanhai Li 1, Yufei Hou 1, Meiyao Feng 1, Ying Wang 1, Jing Chen 1, Jinquan Zhao 1, Yuxin Zheng 2, Dianke Yu 3. Integration of proteomics, lipidomics, and metabolomics reveals novel metabolic mechanisms underlying N, N-dimethylformamide induced hepatotoxicity. Ecotoxicol Environ Saf. 2020 Dec 1:205:111166. doi: 10.1016/j.ecoenv.2020.111166. Epub 2020 Aug 19.
4、Zaichao Xu,Kaitao Zhao,Jingjing Wang,Lu Zhang,Jiatong Yin,Nijing Chen,Sijia Chen,Gaihong Zhao,Mengfei Wang,Tailai Xin,Chengliang Zhu,Xiaoming Cheng ,Yuchen Xia Screening of different species reveals cat hepatocytes support HBV infectionPublished: August 4, 2025 https://doi.org/10.1371/journal.ppat.1013390
Scope of Application
1、Basic Liver Research Encompassing fundamental studies on liver diseases, viral infections, and gene expression regulation.
2、Drug Development: Encompassing studies on drug efficacy, toxicity, metabolism, and drug-drug interactions.
Gene Manipulation Methods
1. Lentivirus, MOI=30-50 can reach close to 100% infection efficiency, need to test in advance;
2, AAV, MOI=10^5 can reach more than 80% infection efficiency, need to be tested in advance;
3, ASGPR-GalNac free uptake, small nucleic acids need to modify GalNac in advance, can achieve more than 80% delivery efficiency;
4, Lipo2000/3000 is not recommended, high toxicity and low efficiency of primary hepatocyte transfection, unless it has been tested without problems.
Due to biannual updates of our product instruction manual, the following procedures are for reference only.
The most recent version included with the product shall prevail.
II Reagents and Materials
- Primary human hepatocytes
- Recovery medium(Cat#LV-Rec001)
- Purified medium(If desired, give with the cells)
- Plating medium(Adherent cells are needed.Cat#LV-WEP001)
- Sustaining medium(Adherent cells are needed. Cat#LV-WEM001)
- Collagen-coated culture plate(Adherent cells are needed.
Cat #LV-Coated)
- Sterile centrifuge tube of 15 ml
- Disposable pipette
- Wide-mouth pipette tip (The tip of a normal one is cut off and sterilized)
- Pipette
-Thermostat water bath (Preheat at 37°. Please calibrate the temperature with a thermometer)
- Refrigerated horizontal centrifuge (with horizontal rotor. it is possible to centrifuge 15 ml of centrifuge tubes)
- Biosafety cabinet
- 37 °C/5% CO2 incubator
IV Resurgence of Adherent Cells
1. In the biosafety cabinet, add 10 ml of resuscitation medium to a 15 ml centrifuge tube and preheat in thermostat water bath of 37 °C for 20 min. Then transfer it to the biosafety cabinet. Purified medium should be placed at room temperature and plating medium should be preheated at 37 °C.
2. Quickly transfer the frozen hepatocytes from the refrigerated position to a thermostat water bath of 37℃, and immerse them in as much water as possible at 37 °C. Shake it clockwise and horizontally for thawing, but make sure that the cap of the freezing tube is kept above the water.
3. Thaw the freezing tube for about 90-120s, until only a small amount of crushed ice floats in it.
4. Sterilize the freezing tube with 75% alcohol and transfer it to a biosafety cabinet.
5. Aspirate the cells with a wide-mouth pipette tip and add them by dropwise to a centrifuge tube containing the preheated resuscitation medium. (Note: if there are more cells left on the freezing tube and the pipette tip, aspirate 1 ml of resuscitation medium to clean the them. Then, mix them into the medium of the centrifuge tube). Slightly turn the tube upside down 2-3 times to mix well.
6. It can be centrifuged of 50 ×g directly at low speed. Then,centrifuge it at room temperature for 5 min. After that, go to the supernatant and resuspend with 4 ml of plating medium. The viability of hepatocytes can be measured with trypan blue exclusion. If the viability > 70%, you can directly plate, otherwise purification is required.
7. If cell purification is required: Centrifuge the cell suspension of 50 × g at room temperature for 5 min. Remove the supernatant and resuspend the cells with 2 ml of purified medium (freely available). Then carefully adds 1 ml of plating medium along the wall to the centrifuge tube (Do not disturb the lower layer, significant delamination can be visible after addition). Centrifuge at 4 °C of 800 × g for 20 min (ascending to 9, downgrading to 1). After centrifugation, aspirate the turbid band (living cell layer) between the purified medium and the plating medium and transfer it to a new centrifuge tube of 15 mL. Add twice volumes of plating medium and mix well. After that, centrifuge of 50 × g for 5 min at room temperature, remove the supernatant and resuspend the cells with 4 ml of plating medium.
8. The survival rate and total number of hepatocytes can be measured by trypan blue exclusion. It is recommended to detect 3 times to obtain the average value.
9. The cells obtained in step 6 should be strictly in accordance with the specific experimental requirements to select the appropriate treatment method.
1) Cell culture: The cell suspension can be directly plated, seeded into collagen-coated plates according to the following density, shaken well, and cultured in a 37℃/5% CO2 incubator:
Medium-density culture: 1.2~1.8 ×105cells/cm2 (average 1.5 ×105cells/cm2). Incubate at 37 °C/5% CO2 incubator for 4-6 h. Theoretically, the degree of cell confluency is about 50-80%.
High-density culture: 1.8~2.0 ×105cells/cm2 (average 1.9 ×105cells/cm2). Incubate at in a 37 °C/5% CO2 incubator for 4-6 h. Theoretically, the degree of cell confluency is about 80-90%.
2) Human-mouse chimeric model: Please centrifuge the cells obtained from step 6 at low speed (50× g, room temperature, 5 min). Go to supernatant and the cell pellet can be resuspended with PBS/normal saline/medium. Then, the cell transplantation should be performed as soon as possible.
V Hepatocyte Maintenance and Hepatitis B virus Infection
1. Adhere to the culture for 4-6 h, the cells have been fully adherent. Then, remove the plate medium (including some dead cells), and add the sustaining medium. The solution should be changed every 2 days.
2. Preparation of infection culture: 4% PEG8000 should be pre-added to the sustaining medium and mixed well. HBV virus (HepAD38 MOI =500 to 1000, purified patient serum MOI =100 to 500, higher MOI may further improve infection efficiency).
3. Remove the old culture medium, add the infection culture medium, and incubate in a 37 °C/5% CO2 incubator for 16 h or overnight.
4. Remove the infection culture, PBS wash 3 times, add sustaining medium, and continue the culture in a 37 °C/5% CO2 incubator.
After HBV infection, the supernatant is collected once every 2 days for viral antigen and HBV DNA detection. Collect the supernatants of 3dpi, 5dpi, 7dpi, 9dpi, and 9dpi can end cell culture. Longer culture times depend on cell status (dpi: number of days of culture after infection).
Post-Thaw Cells Must Not Undergo Refreezing.
VI Customer Service
If you find any quality problems with the product, please collect the original data and contact the company's salesmen or technical support at the first time. The company ensure after-sales service. Every laboratory has different conditions, different operating habits, different proficiency, and objective factors in experimental failure. If the operation is not strictly in accordance with the instructions or exceeds the time limit of after-sales, the company does not do after-sales. Please understand and support us.
Validity period and raw data provided:
Resuscitation problems: Within 24 hours of resuscitation, trypan blue staining or PI staining should be provided.
Pollution problems: Within 96 hours of resuscitation, microscope photographs of differences should be provided.
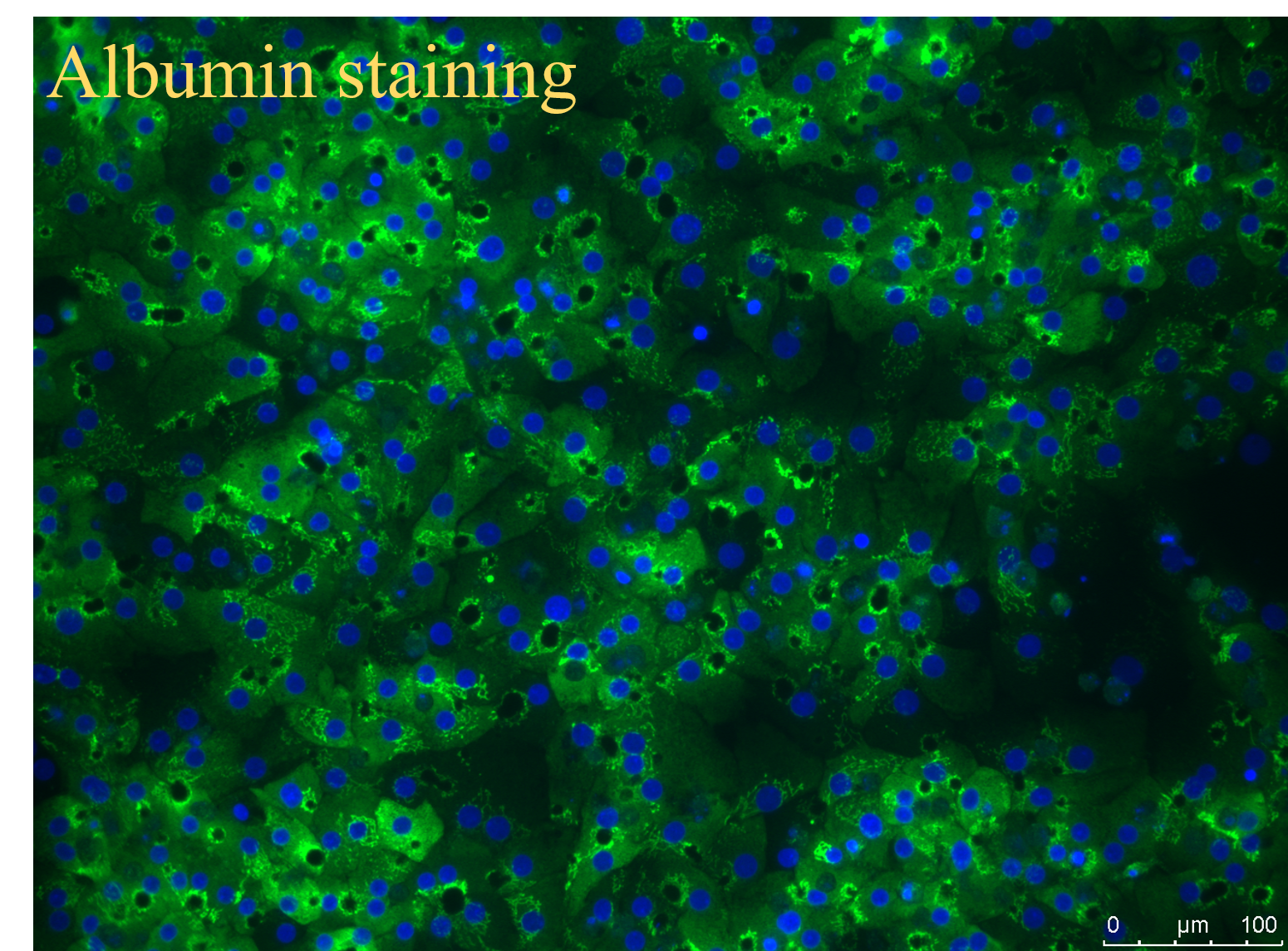
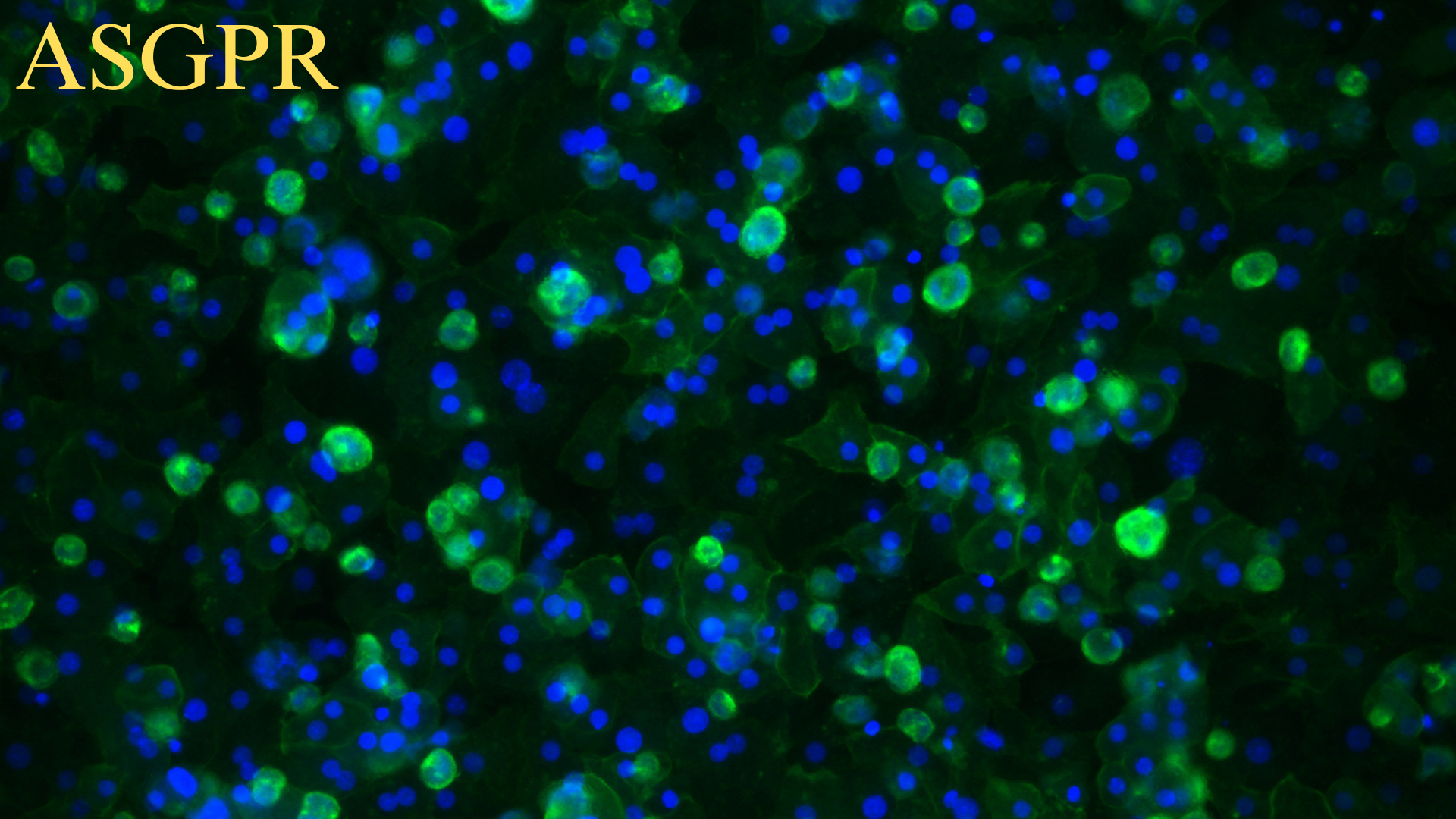
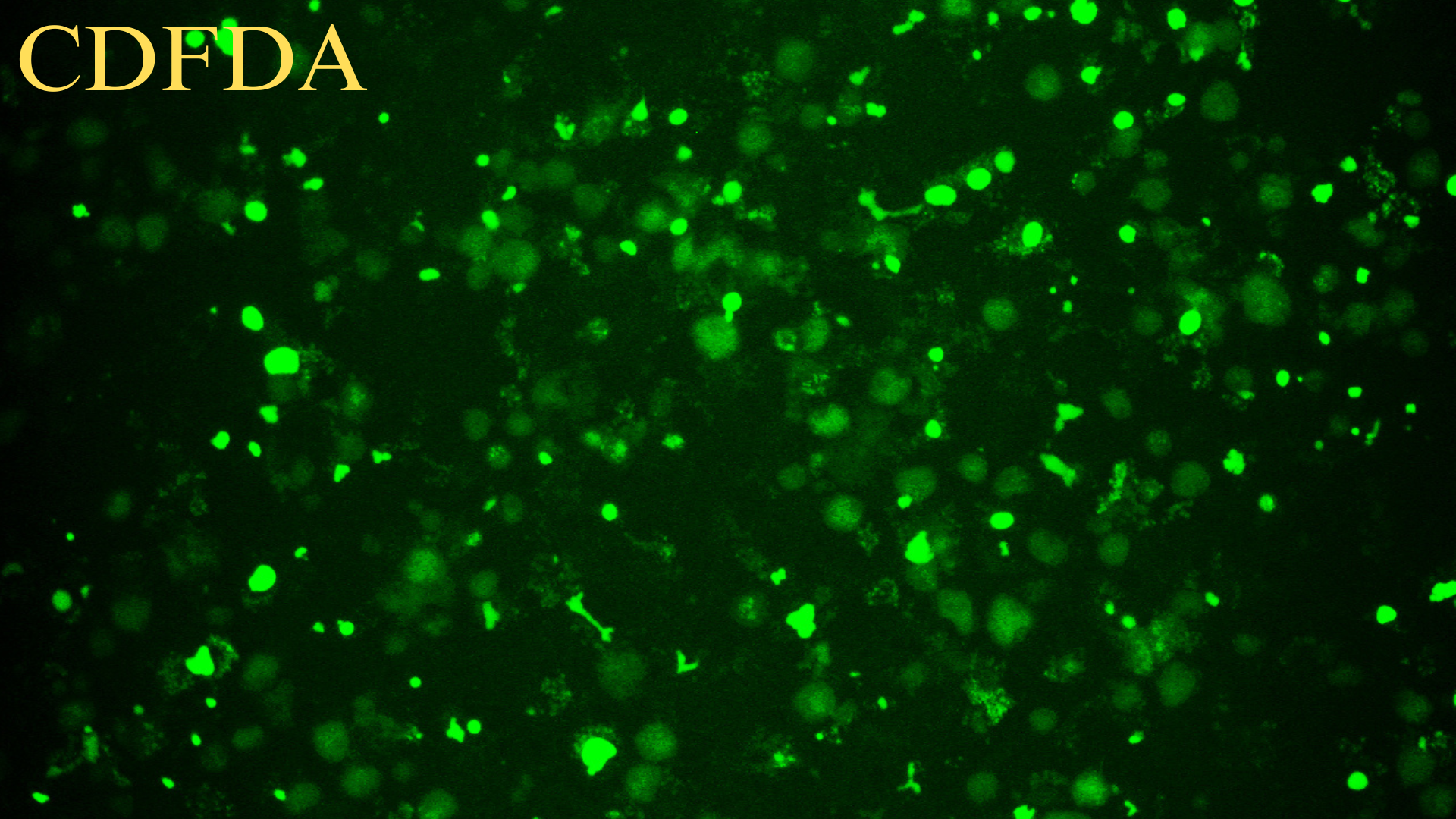
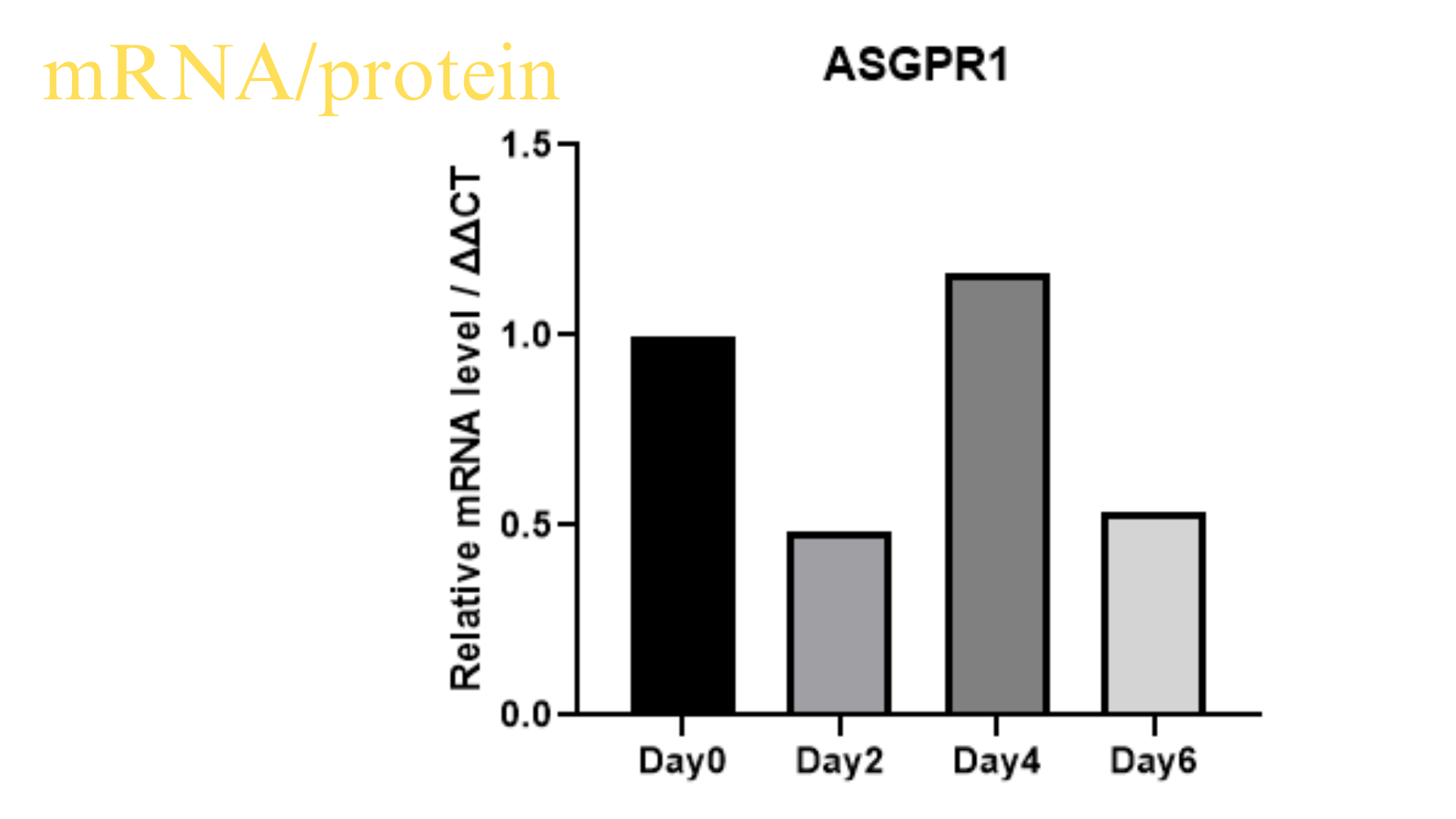
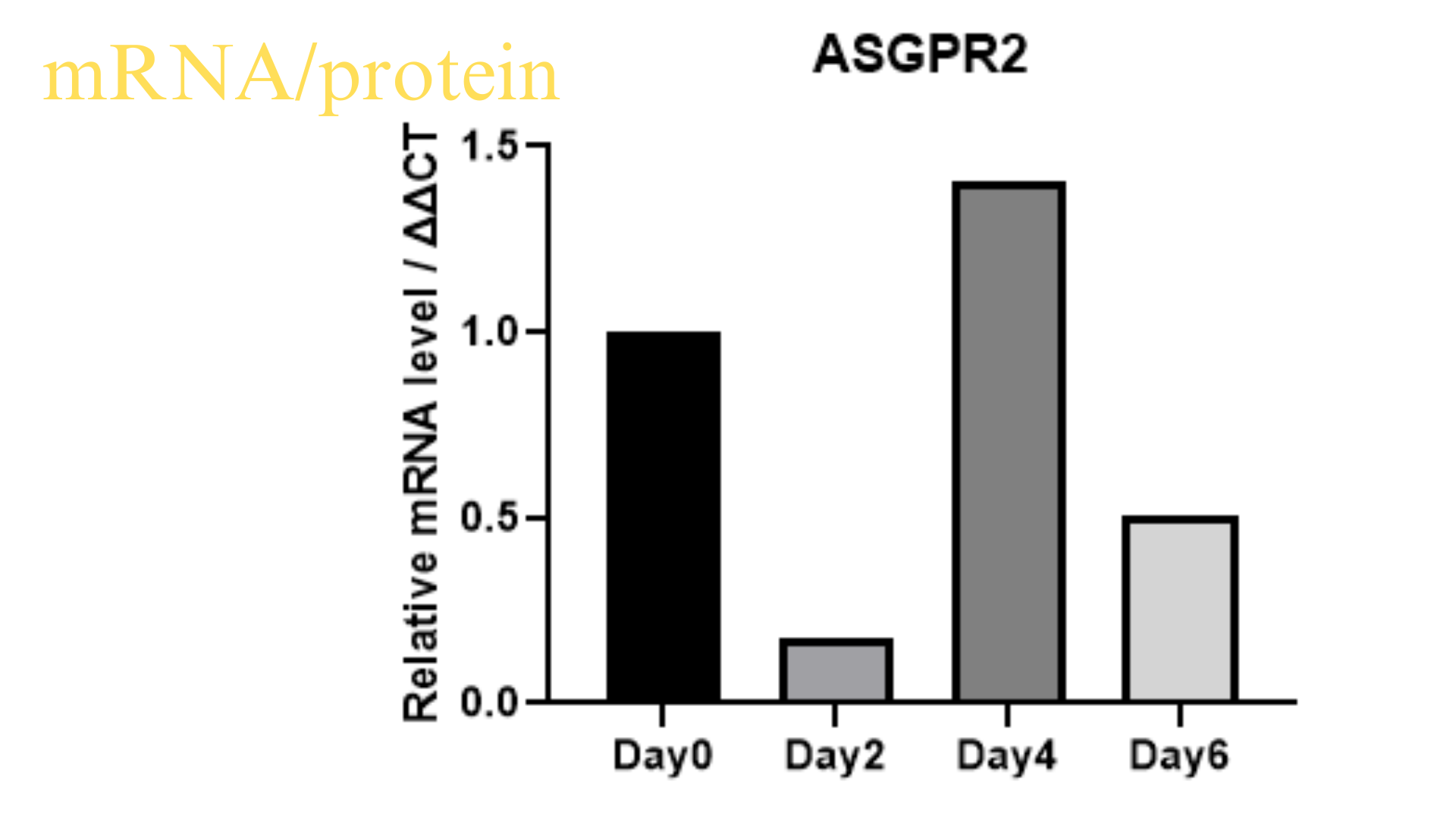
Purity issues: Within one month, immunofluorescence or flow cytometry results should be provided.
VII Contact Number
Tel:0755-28284050
Technical Support: 19902901483 (Dr. Zhou)